CLINIC DIRECTOR
Director’s Message
Kiyoshi Yokokawa
Since its founding in April 2012, the Kobe International Cell Therapy Center has been dedicated to cancer immunotherapy for over 13 years.
Throughout this time, we have remained committed to a patient-centered philosophy, integrating our original treatment approaches to deliver a wide variety of personalized, tailored therapies for cancer patients.
About seven years ago, Kyoto University in Japan took the lead in conducting pioneering experiments and research on cancer immunotherapy.
In collaboration with a Japanese pharmaceutical company, they successfully developed and mass-produced the world’s first immune checkpoint inhibitor (ICI: Immuno-checkpoint Inhibitor).
As many may know, this breakthrough profoundly reshaped the landscape of cancer treatment.
At the same time, the emergence of ICI had a deep and far-reaching impact on our own medical philosophy and practice.It can be said that ICI was one of the key catalysts that inspired us to launch our Mesenchymal Stem Cell Therapy program in June 2021.
It is worth highlighting that mesenchymal stem cells (MSCs) have shown remarkable potential in treating a wide range of diseases.
These include motor dysfunction resulting from brain hemorrhage, stroke, and spinal cord injury;
degenerative knee osteoarthritis caused by aging or physical overuse; and, more recently, conditions closely linked to chronic inflammation, such as liver dysfunction and diabetes, which have drawn increasing clinical attention in recent years.
The therapeutic mechanisms of mesenchymal stem cells (MSCs) are primarily driven by the diverse range of growth factors they secrete, which can effectively regulate and suppress the immune system. These cells also promote angiogenesis—the formation of new blood vessels—enhancing the body’s overall repair and regulatory capacity.
In daily life, chronic stress often triggers subtle physiological imbalances, many of which stem from persistent, low-grade inflammation. MSCs, which are stored in adipose (fat) tissue and circulate throughout the body, possess the ability to regulate these unnecessary inflammatory responses.
This powerful anti-inflammatory capacity may hold the key to maintaining overall health, delaying aging, and ultimately promoting longevity.
| 1978.03 | Graduated from Osaka University School of Medicine |
|---|---|
| 1987 | Obtained Ph.D. from Osaka University |
| 1993.07 | Joined Sando Pharmaceutical Co., Ltd. (now Novartis Pharma K.K.) |
| 1997.04 | Joined Takeda Pharmaceutical Company Limited |
| 1998.10 | Head of Oncology, Urology, and Infectious Diseases Department at Takeda |
| 2004.02 | Joined Medinet Co., Ltd. |
| 2004.12 | Director & CSO of R&D at Medinet |
| 2007.04 | Representative Director & COO of Medinet |
| 2010.11 | Appointed Chief Director of Medical Corporation Ishokukai |
| 2012.04 | Opened Kobe International Cell Therapy Center |
Facilities

Entrance

Reception

Examination Room
Administration Room
Treatment Room

Conference Room

Corridor

CPC Room
(Cell Processing Center)

Restroom
Our Strengths
Japan’s longest-standing regenerative medicine institute, with 13+ years of expertise in stem and immune cell therapies. Founded in 2012, we lead the field with rich clinical experience.
In-house CPC Cell Culture Center – Safe and Efficient
We manage each step—from cell extraction to storage—to ensure quality, safety, and consistent treatment results for every patient.
Powered by Innovation and Technology
We use advanced regenerative technologies to continually refine therapies, delivering safer and more effective care tailored to patient needs.
Experienced Care with High Patient Satisfaction
Since 2012, we have built a strong clinical record with thousands of cases, earning high patient satisfaction and strong trust.
CERTIFICATION
Stem Cell Certificates
| Cell Culture Processing Facility (CPC) Licensed by Japan Ministry of Health, Labour and Welfare |
| NB5150011 |
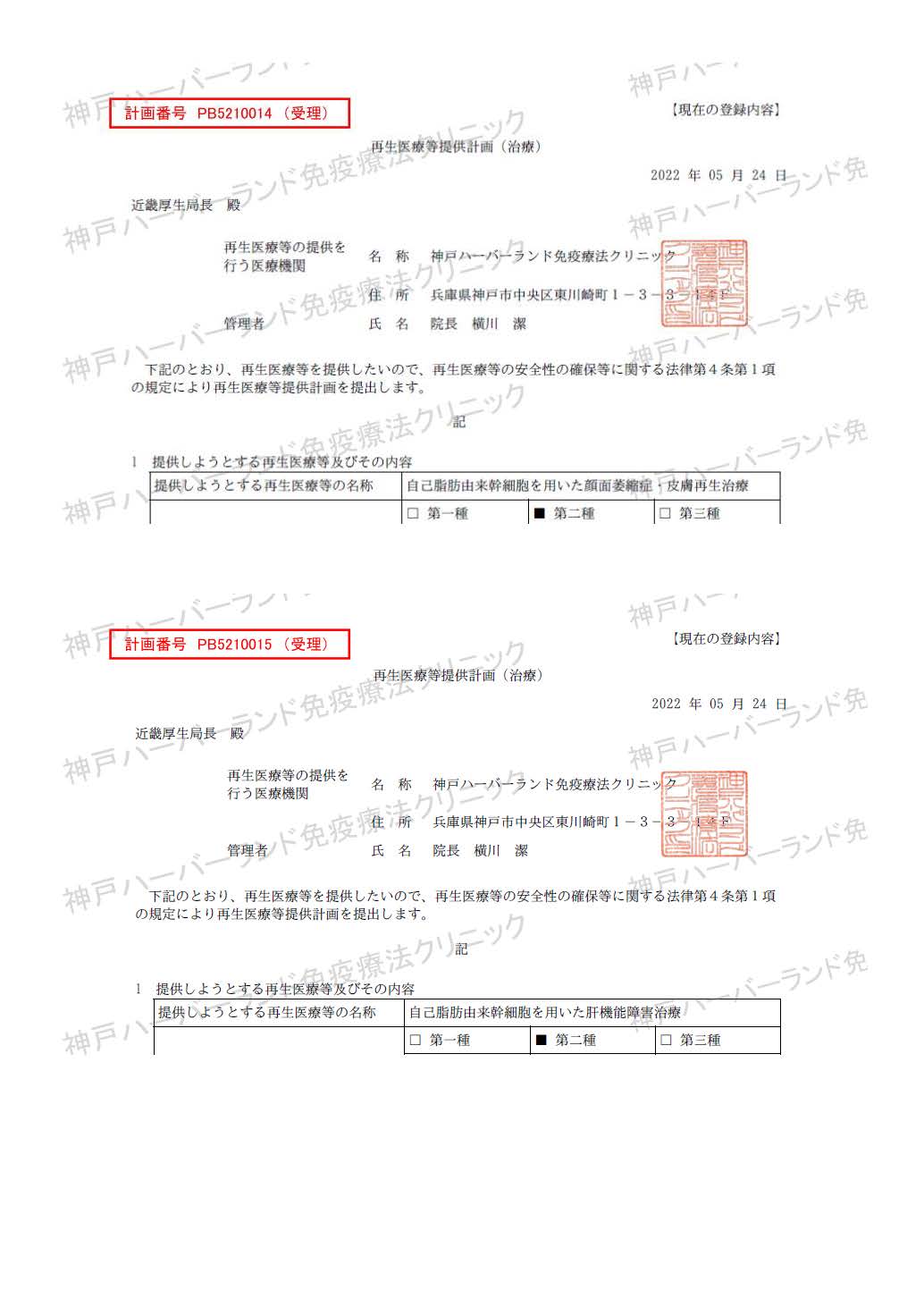
| Submitted Regenerative Medicine Provision Plans |
| Kobe Harborland immunotherapy clinic is a medical institution that has submitted second- and third-class regenerative medicine provision plans to the Japan Ministry of Health, Labour and Welfare and obtained plan numbers. |
| Name of Regenerative Medicine | License Number |
|---|---|
| Treatment of diabetes and pre-diabetes using autologous adipose-derived stem cells | PB5240108 |
| Treatment of liver dysfunction using autologous adipose-derived stem cells | PB5210015 |
| Treatment of facial atrophy and skin regeneration with autologous adipose-derived stem cells | PB5210014 |
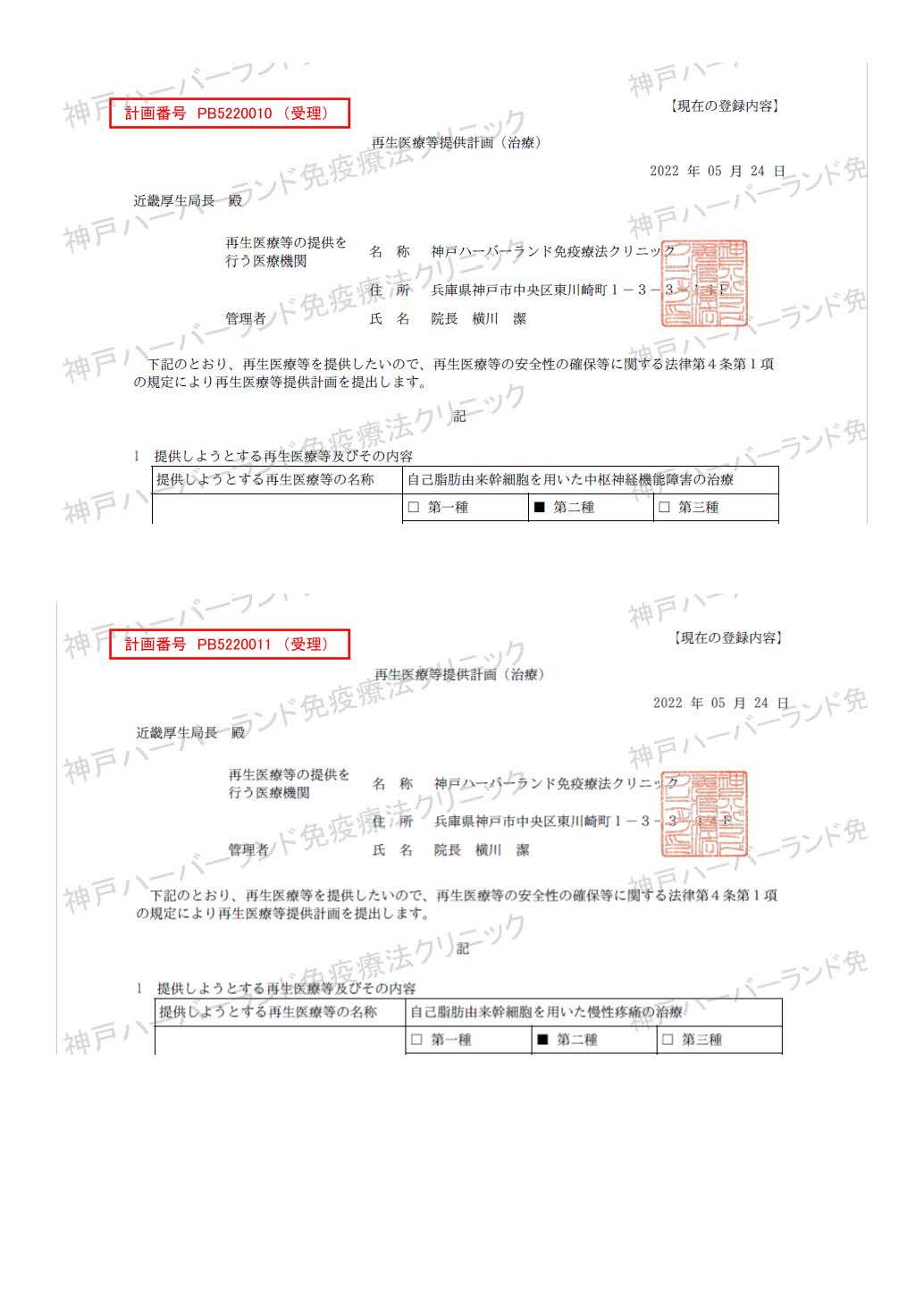
| Treatment of central nervous system dysfunction using autologous adipose-derived stem cells | PB5220010 |
| Treatment of chronic pain using autologous adipose-derived stem cells | PB5220011 |
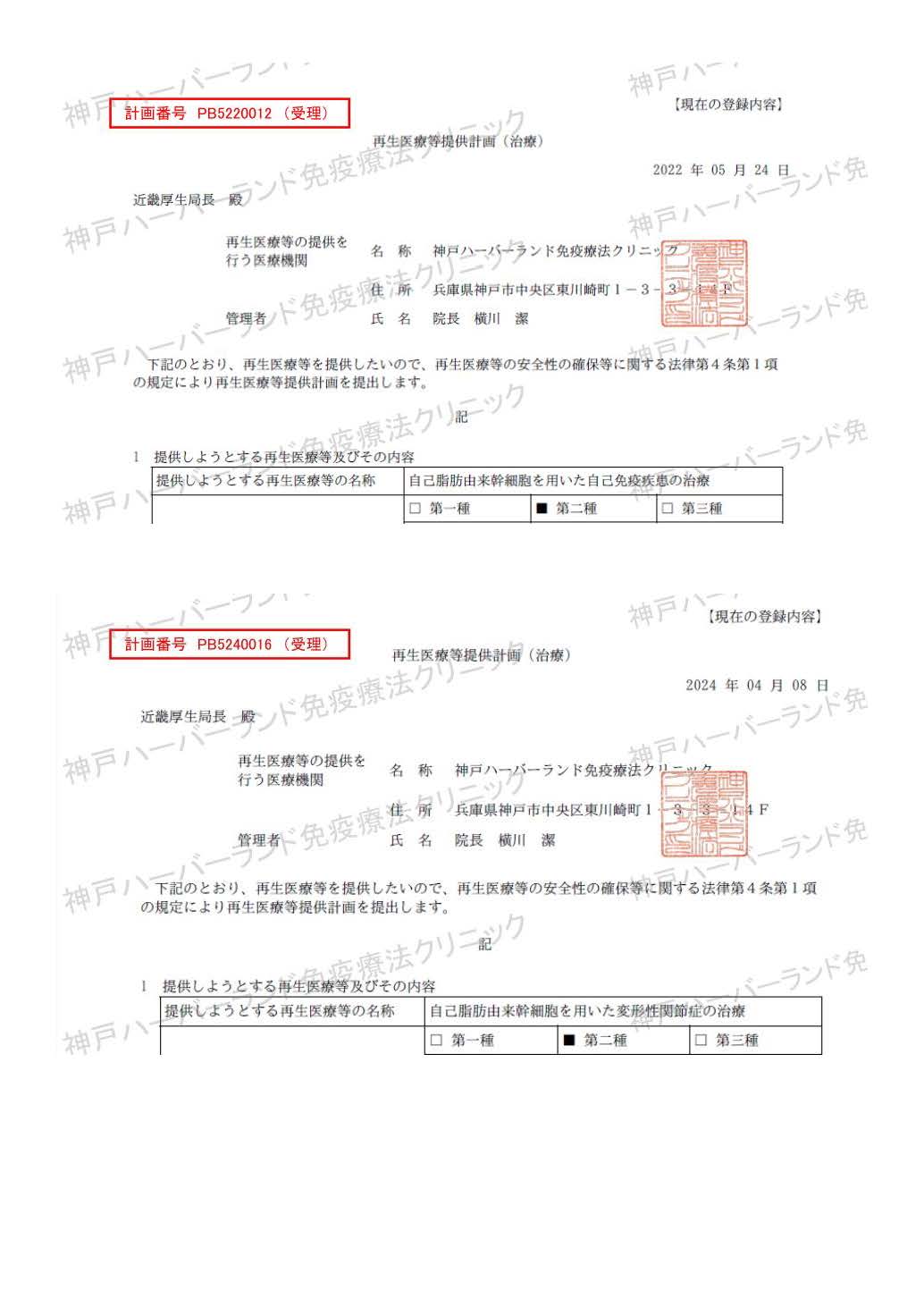
| Treatment of autoimmune diseases using autologous adipose-derived stem cells | PB5220012 |
| Treatment of osteoarthritis using autologous adipose-derived stem cells | PB5240016 |
| Dendritic cell vaccine therapy for the treatment and prevention of recurrence of malignant neoplasms | PC5150140 |
| Activated T lymphocyte therapy for the treatment and prevention of recurrence of malignant neoplasms | PC5150138 |
| Natural killer (NK) cell therapy for the treatment and prevention of recurrence of malignant neoplasms | PC5150139 |
| NKT cell therapy using dendritic cells for the treatment and prevention of recurrence of malignant tumors | PC5190043 |
2.png)